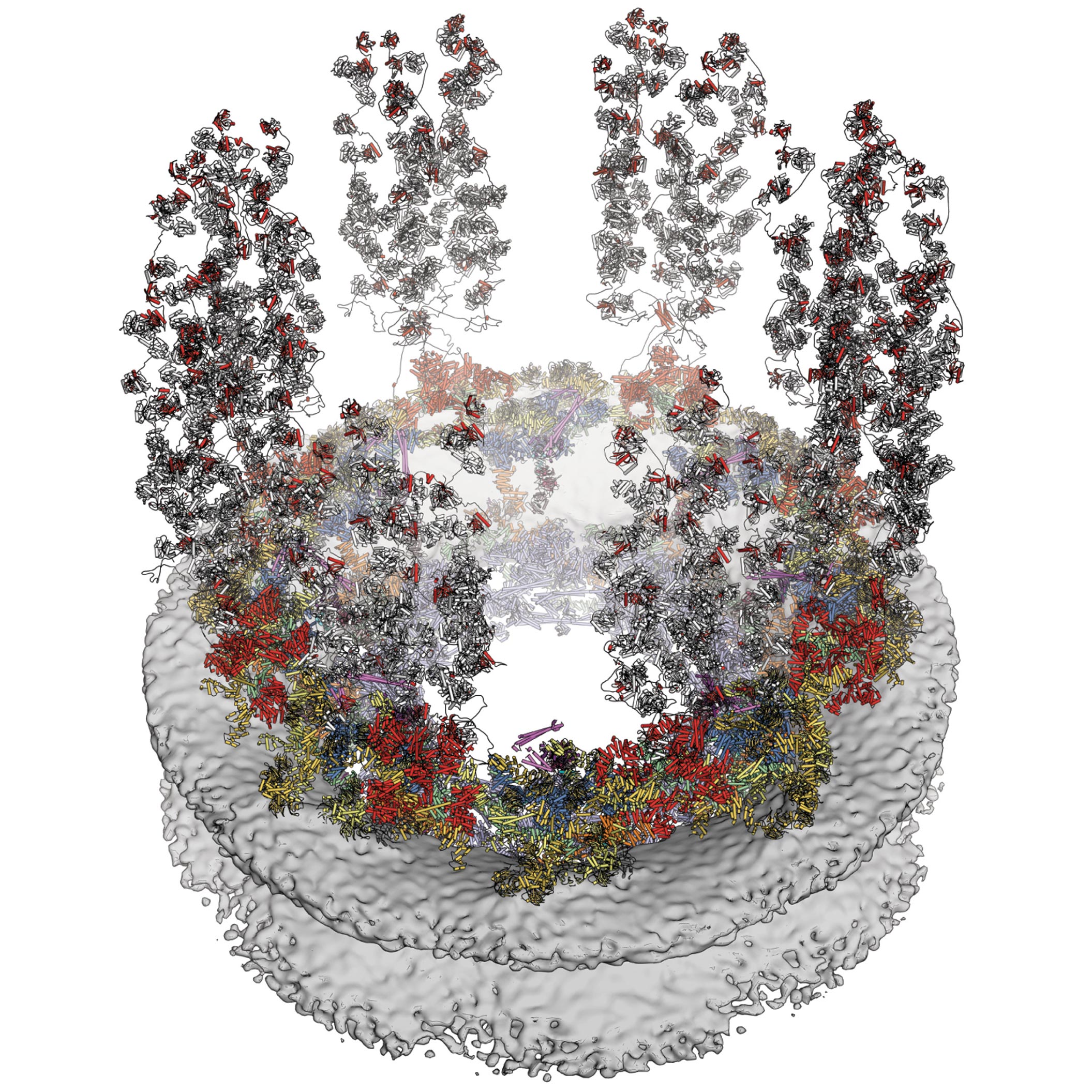
Scientists are deciphering the nuclear pore complex in fabulous detail. Credit rating: Valerie Altounian
Different us learned the final cell structure at some level and will expend formula love the cell membrane, cytoplasm, mitochondrion, and nucleus. Nonetheless, the structure of our cells is de facto vastly extra complex than you would have belief. Truly, because now we had been discovering so unparalleled through the years, we now know that cells are a ways extra complex than even expert biologists realized now not too lengthy ago.
One component of particular complexity is the nuclear pore complex. Surrounding the eukaryotic cell nucleus is a double membrane, the nuclear envelope, which encloses the genetic subject cloth of the cell nucleus. Spanning that nuclear envelope is the nuclear pore complex, which though little in dimension, is extremely complex molecular equipment created from a immense series of varied proteins.
Whatever you would also very wisely be doing, whether it’s miles riding a car, going for a fling, or even at your laziest, drinking chips and staring at TV on the sofa, there would possibly be a total suite of molecular equipment inner every of your cells laborious at work. That equipment, a ways too small to perceive with the naked tag or even with many microscopes, creates vitality for the cell, manufactures its proteins, makes copies of its DNA, and a ways extra.
Amongst those items of equipment, and if truth be told one of basically the most complex, is something frequently called the nuclear pore complex (NPC). The NPC, which is created from extra than 1,000 individual proteins, is an extremely discriminating gatekeeper for the cell’s nucleus, the membrane-certain self-discipline inner a cell that holds that cell’s genetic subject cloth. Anything getting into into or out of the nucleus has to pass thru the NPC on its plan.
A molecular mannequin of the skin (cytoplasmic) face of the nuclear pore complex. Reprinted with permission from C.J. Bley et al., Science 376, eabm9129 (2022). Credit rating: Hoelz Laboratory/Caltech
The NPC’s feature as a gatekeeper of the nucleus plan it’s miles needed for the operations of the cell. Inner the nucleus, DNA, the cell’s everlasting genetic code, is copied into RNA. That RNA is then implemented of the nucleus so it would even be historical to bear the proteins the cell desires. The NPC ensures the nucleus gets the materials it desires for synthesizing RNA, while also retaining the DNA from the cruel ambiance outside the nucleus and enabling the RNA to go the nucleus after it has been made.
“It’s honest a diminutive of love an airplane hangar where you would repair 747s, and the door opens to let the 747 are accessible in, nonetheless there’s a particular person standing there who can preserve a single marble from getting out while the doorways are inaugurate,” says Caltech’s André Hoelz, professor of chemistry and biochemistry and a Faculty Student of the Howard Hughes Scientific Institute. For added than two many years, Hoelz has been finding out and deciphering the structure of the NPC in relation to its feature. Over time, he has continuously chipped away at its secrets and tactics, unraveling them fragment by fragment by fragment by fragment.
The implications of this review are potentially tall. Not finest is the NPC central to the operations of the cell, moreover it’s miles eager with many diseases. Mutations in the NPC are accountable for some incurable cancers, for neurodegenerative and autoimmune diseases such as amyotrophic lateral sclerosis (ALS) and acute necrotizing encephalopathy, and for coronary heart prerequisites including atrial fibrillation and early unexpected cardiac dying. Furthermore, many viruses, including the one accountable for COVID-19, target and shutdown the NPC at some stage in the route of their lifecycles.
Now, in a pair of papers printed in the journal Science, Hoelz and his review group describe two vital breakthroughs: the resolution of the structure of the outer face of the NPC and the elucidation of the mechanism by which special proteins act love a molecular glue to preserve the NPC collectively.
A extraordinarily diminutive 3D jigsaw puzzleIn their paper titled “Architecture of the cytoplasmic face of the nuclear pore,” Hoelz and his review group describe how they mapped the structure of the aspect of the NPC that faces outward from the nucleus and into the cells’ cytoplasm. To enact this, they needed to solve the equal of a really diminutive 3-d jigsaw puzzle, using imaging tactics such as electron microscopy and X-ray crystallography on every puzzle fragment.
Stefan Petrovic, a graduate student in biochemistry and molecular biophysics and if truth be told one of many co-first authors of the papers, says the contrivance began with Escherichia coli micro organism (a force of micro organism assuredly historical in labs) that had been genetically engineered to kind the proteins that kind up the human NPC.
“Whereas you stroll into the lab, you would rely on this enormous wall of flasks by which cultures are rising,” Petrovic says. “We teach every individual protein in E. coli cells, spoil those cells inaugurate, and chemically purify every protein component.”
Once that purification—which would per chance require as unparalleled as 1,500 liters of bacterial culture to obtain adequate subject cloth for a single experiment—was once total, the review group began to painstakingly take a look at how the items of the NPC match collectively.
George Mobbs, a senior postdoctoral scholar review partner in chemistry and yet every other co- first writer of the paper, says the assembly took train in a “stepwise” vogue; relatively than pouring the total proteins collectively into a take a look at tube on the identical time, the researchers tested pairs of proteins to perceive which of them would match collectively, love two puzzle items. If a pair was once learned that match collectively, the researchers would then take a look at the 2 now-combined proteins in opposition to a 3rd protein till they learned one which match with that pair, and then the ensuing three-fragment structure was once tested in opposition to other proteins, and tons others. Working their plan thru the proteins in this vogue sooner or later produced the final results of their paper: a 16-protein wedge that is repeated eight times, love slices of a pizza, to kind the face of the NPC.
“We reported the first total structure of the total cytoplasmic face of the human NPC, along with rigorous validation, relatively than reporting a series of incremental advances of fragments or portions per partial, incomplete, or low-resolution commentary,” says Si Nie, postdoctoral scholar review partner in chemistry and also a co-first writer of the paper. “We determined to patiently wait till we had bought all needed data, reporting a humungous quantity of unusual data.”
Their work complemented review conducted by Martin Beck of the Max Planck Institute of Biophysics in Frankfurt, Germany, whose group historical cryo-electron tomography to generate a diagram that provided the contours of a puzzle into which the researchers needed to train the items. To bustle up the completion of the puzzle of the human NPC structure, Hoelz and Beck exchanged data extra than two years ago and then independently constructed constructions of the total NPC. “The critically improved Beck diagram confirmed unparalleled extra clearly where every fragment of the NPC—for which we determined the atomic constructions—needed to be positioned, such as a wooden frame that defines the perimeter of a puzzle,” Hoelz says.
The experimentally determined constructions of the NPC items from the Hoelz personnel served to validate the modeling by the Beck personnel. “We positioned the constructions into the diagram independently, using varied approaches, nonetheless the final results fully agreed. It was once very nice to perceive that,” Petrovic says.
“We constructed a framework on which loads of experiments can now be done,” says Christopher Bley, a senior postdoctoral scholar review partner in chemistry and also co-first writer. “We have now this composite structure now, and it enables and informs future experiments on NPC feature, or even diseases. There are loads of mutations in the NPC which are associated with dreadful diseases, and nice looking where they are in the structure and the plan in which they come collectively can abet kind the subsequent feature of experiments to take hold of a see at and reply the questions of what these mutations are doing.”
“This trim diagram of spaghetti noodles”Within the opposite paper, titled “Architecture of the linker-scaffold in the nuclear pore,” the review group describes the plan in which it determined the total structure of what’s legendary as the NPC’s linker-scaffold—the series of proteins that abet preserve the NPC collectively while also offering it with the flexibleness it desires to inaugurate and shut and to alter itself to study the molecules that pass thru.
Hoelz likens the NPC to something constructed out of Lego bricks that match in conjunction with out locking collectively and are as an substitute lashed collectively by rubber bands that preserve them largely in train while level-headed allowing them to pass around honest a diminutive.
The nuclear pore complex (NPC) is able to kind bigger and contract to adapt to the desires of the cell. Reprinted with permission from S. Petrovic et al., Science 376, eabm9798 (2022). Credit rating: Hoelz Laboratory/Caltech
“I call these unstructured glue items the ‘darkish subject of the pore,’” Hoelz says. “This trim diagram of spaghetti noodles holds everything collectively.”
The process for characterizing the structure of the linker-scaffold was once unparalleled the identical as the contrivance historical to describe the opposite parts of the NPC. The group manufactured and purified huge amounts of the many styles linker and scaffold proteins, historical a diversity of biochemical experiments and imaging tactics to gaze individual interactions, and tested them fragment by fragment to perceive how they match collectively in the intact NPC.
To review their work, they launched mutations into the genes that code for every of those linker proteins in a residing cell. Since they knew how those mutations would substitute the chemical properties and form of a teach linker protein, making it putrid, they would well predict what would happen to the structure of the cell’s NPCs when those putrid proteins had been launched. If the cell’s NPCs had been functionally and structurally putrid in the style they expected, they knew they had the ethical diagram of the linker proteins.
“A cell is plan extra complex than the easy system we originate in a take a look at tube, so it’s miles extremely most important study that results received from in vitro experiments preserve up in vivo,” Petrovic says.
The assembly of the NPC’s outer face also helped solve a longtime thriller relating to the nuclear envelope, the double membrane system that surrounds the nucleus. Just like the membrane of the cell inner which the nucleus resides, the nuclear membrane is now not perfectly snug. Quite, it’s miles studded with molecules called integral membrane proteins (IMPs) that again in a diversity of roles, including performing as receptors and serving to to catalyze biochemical reactions.
Although IMPs would per chance even be learned on every the internal and outer facets of the nuclear envelope, it had been unclear how they genuinely traveled from one aspect to the opposite. Certainly, because IMPs are caught inner of the membrane, they can now not upright waft thru the central transport channel of the NPC as enact free-floating molecules.
Once Hoelz’s group understood the structure of the NPC’s linker-scaffold, they realized that it enables for the formation of diminutive “gutters” around its outside edge that permit the IMPs to traipse previous the NPC from one aspect of the nuclear envelope to the opposite while consistently staying embedded in the membrane itself.
“It explains loads of issues which had been enigmatic in the self-discipline. I am very ecstatic to perceive that the central transport channel indeed has the power to dilate and kind lateral gates for these IMPs, as we had originally proposed extra than a decade ago,” Hoelz says.
Taken collectively, the findings of the 2 papers symbolize a soar forward in scientists’ working out of how the human NPC is constructed and the plan in which it surely works. The group’s discoveries inaugurate the door for a ways extra review. “Having determined its structure, we are able to now level of curiosity on working out the molecular bases for the NPC’s capabilities, such as how mRNA gets exported and the underlying causes for the many NPC-associated diseases with the purpose of increasing unusual therapies,” Hoelz says.
The papers describing the work seem in the June 10 mission of the journal Science.
References:
“Architecture of the cytoplasmic face of the nuclear pore” by Christopher J. Bley, Si Nie, George W. Mobbs, Stefan Petrovic, Anna T. Gres, Xiaoyu Liu, Somnath Mukherjee, Sho Harvey, Ferdinand M. Huber, Daniel H. Lin, Bonnie Brown, Aaron W. Tang, Emily J. Rundlet, Ana R. Correia, Shane Chen, Saroj G. Regmi, Taylor A. Stevens, Claudia A. Jette, Mary Dasso, Alina Patke, Alexander F. Palazzo, Anthony A. Kossiakoff and André Hoelz, 10 June 2022, Science.
DOI: 10.1126/science.abm9129
“Architecture of the linker-scaffold in the nuclear pore” by Stefan Petrovic, Dipanjan Samanta, Thibaud Perriches, Christopher J. Bley, Karsten Thierbach, Bonnie Brown, Si Nie, George W. Mobbs, Taylor A. Stevens, Xiaoyu Liu, Giovani Pinton Tomaleri, Lucas Schaus and André Hoelz, 10 June 2022, Science.
DOI: 10.1126/science.abm9798
Further co-authors of the paper, “Architecture of the cytoplasmic face of the nuclear pore,” are Anna T. Gres; now of Worldwide Scientific Trials; Xiaoyu Liu, now of UCLA; Sho Harvey, a worn grad student in Hoelz’s lab; Ferdinand M. Huber, now of Odyssey Therapeutics; Daniel H. Lin, now of the Whitehead Institute for Biomedical Be taught; Bonnie Brown, a worn review technician in Hoelz’s lab; Aaron W. Tang, a worn review technician in Hoelz’s lab; Emily J. Rundlet, now of St. Jude Young folks’s Be taught Clinic and Weill Cornell Medication; Ana R. Correia, now of Amgen; Taylor A. Stevens, graduate student in biochemistry and molecular biophysics; Claudia A. Jette, graduate student in biochemistry and molecular biophysics; Alina Patke, review assistant professor of biology; Somnath Mukherjee and Anthony A. Kossiakoff of the College of Chicago; Shane Chen, Saroj G. Regmi, and Mary Dasso of the National Institute of Diminutive one Health and Human Pattern; and Alexander F. Palazzo of the College of Toronto.
Further co-authors of the paper, “Architecture of the linker-scaffold in the nuclear pore,” are Dipanjan Samanta, postdoctoral scholar fellowship trainee in chemical engineering; Thibaud Perriches, now of Care Companions; Christopher J. Bley; Karsten Thierbach; now of Odyssey Therapeutics; Bonnie Brown, Si Nie, George W. Mobbs, Taylor A. Stevens, Xiaoyu Liu, now of UCLA; Giovani Pinton Tomaleri, graduate student in biochemistry and molecular biophysics; and Lucas Schaus, graduate student in biochemistry and molecular biophysics.
Funding for the review was once provided by the National Institutes of Health, the Howard Hughes Scientific Institute, and the Heritage Scientific Be taught Institute.