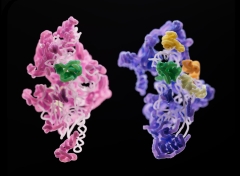
A subunit of a yeast mitoribosome (pink) compared to that of a human mitoribosome (purple). Various, the 2 establishing subunits have an assembly element (green) in typical. Credit: Sebastian Klinge Across the tree of life, ribosomes, the small protein-producing factories within cells, are common and look mainly similar. Ribosomes that keep germs downing along are, structurally, very little various from those producing proteins in our own human cells. Even 2 organisms with comparable ribosomes might show substantial structural distinctions in the RNA and protein elements of their mitoribosomes. Specialized ribosomes within the mitochondria (the energy-producing entities within our cells), mitoribosomes assist the mitochondria fruit and vegetables proteins that make ATP, the energy currency of the cell. The mitochondrial ribosome, or mitoribosome, is a protein complex that is active in mitochondria and operates as a riboprotein for equating mitochondrial messenger RNAs (mRNAs) encoded in mitochondrial DNA (mtDNA). The mitoribosome is connected to the inner mitochondrial membrane. Researchers in the lab of Sebastian Klinge questioned how mitoribosomes progressed, how they put together within the cell, and why their structures are a lot less consistent throughout types. To respond to these concerns, they utilized cryo-electron microscopy to create 3D pictures of the little subunits of yeast and human mitoribosomes as they were being put together. Their findings, which will be released today (December 8) in the journal Nature, clarified the basics of mitoribosome assembly, and might have ramifications for uncommon illness connected to malfunctioning mitoribosomes. “Three-dimensional photos can inform us a lot about what actions are needed, what proteins are associated with the procedure, and how you may be able to control the assembly of these big and intricate makers,” states Nathan Harper, a college student in Klinge’s laboratory. “Cryo-EM permitted us to determine and separate specific phases of the assembly path from a heterogeneous population of cleansed complexes, and we have the ability to see how these complexes alter gradually throughout assembly,” includes Chloe Burnside, likewise a college student in Klinge’s laboratory. By observing this procedure in 2 various types– yeast and people– the group handled to straight observe numerous resemblances and distinctions in mitoribosome assembly. One essential difference: various proteins frequently were associated with otherwise comparable acts of RNA folding. That’s most likely since “there prevail difficulties for these ribosomes,” Harper describes. “You can think of it like producing 2 various bikes– a roadway bike and a mtb. You may require extra parts or tools for each one, however some essential phases in production will be comparable.” The outcomes supply distinct insights into how molecular intricacy and variety occurs in macromolecular complexes, and how assembly systems develop in addition to the complexes themselves. A much better understanding of mitoribosomes might likewise have ramifications for a variety of serious illness connected to mitoribosome dysfunction, such as Perrault syndrome. “We had the ability to map numerous disease-causing anomalies onto various assembly aspects’ structures, so that we might see how these anomalies might impact the ribosome assembly procedure.” Recommendation: “Principles of mitoribosomal little subunit assembly in eukaryotes” 8 December 2022, Nature. DOI: 10.1038/ s41586-022-05621 -0
Read More
Mitoribosome Assembly: How a Cell’s Mitochondria Make Their Own Protein Factories