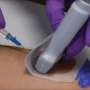
With UltraDrape, ultrasound gel is used to a detachable movie layer on the back of the dressing, avoiding the probe and gel from entering direct contact with the insertion website. Credit: PICC Excellence
A distinct sterilized dressing can enhance client security throughout ultrasound-guided peripheral IV (UGPIV) insertions by helping with a standardized method to aseptic method, offering a barrier versus contamination and guaranteeing efficient securement and dressing adherence, according to a brand-new pilot research study by PICC Excellence and Sharp Healthcare (San Diego).
In this multi-center qualitative observational examination, private clinician actions were gathered instantly following 210 distinct UGPIV treatments in which the sterilized transparent barrier and securement dressing (UltraDrape Parker Laboratories) was utilized rather of a sterilized probe sheath cover for probe defense.
Clinicians discovered the sterilized barrier dressing enhanced ultrasound-guided insertions and was user friendly, with 98 percent of clinicians revealing a choice for UltraDrape over sterilized probe covers. In addition, 99 percent of clinicians suggested embracing the brand-new dressing to standardize UGPIV catheter insertions.
“UGPIV treatments are quicker, more secure, and more economical with efficient standardization,” stated research study co-author and PICC Excellence CEO Nancy Moureau, REGISTERED NURSE, Ph.D., CRNI, CPUI, VA-BC. “Standardizing the treatment with UltraDrape will not just make the UGPIV treatment more secure, however the enhanced performance and lower expenses might likewise allow more clients to take advantage of ultrasound-guided vascular gain access to.”
Almost 60 percent of clients requiring intravenous medication are thought about to have hard vascular gain access to (DiVA) and need ultrasound assistance to attain effective peripheral vascular gain access to. It is approximated that there are 12 million UGPIV insertions carried out each year in North America.
UltraDrape makes sure aseptic strategy with consistency following insertion policies, while preserving sterility at the insertion website wit