The addition of nivolumab to rucaparib did not result in much better progression-free survival than rucaparib monotherapy in clients with freshly identified, advanced, state-of-the-art ovarian cancer (AHGOC), a brand-new research study has actually discovered.
Bradley J. Monk, MD, provided this finding and other outcomes of the ATHENA-COMBO research study, at the European Society for Medical Oncology Congress 2024.
The stage 3, randomized trial compared the effectiveness of rucaparib (Rubraca) plus nivolumab (Opdivo) mix treatment vs rucaparib monotherapy as upkeep treatment in clients with AHGOC who had actually reacted to first-line platinum-based chemotherapy. The research study revealed the mix treatment was connected with numerically much shorter typical PFS compared to rucaparib monotherapy in the intent-to-treat (ITT) population (15.0 months vs. 20.2 months; threat ratio [HR]1.3; 95% CI, 1.1 – 1.5). This pattern corresponded throughout numerous subgroups, consisting of those based upon homologous recombination shortage (HRD) status and configured death-ligand 1 (PD-L1) expression levels.
Throughout his talk, Monk, of the Florida Cancer Specialists & & Research Institute, West Palm Beach, Florida, discussed the reasoning for carrying out ATHENA-COMBO.
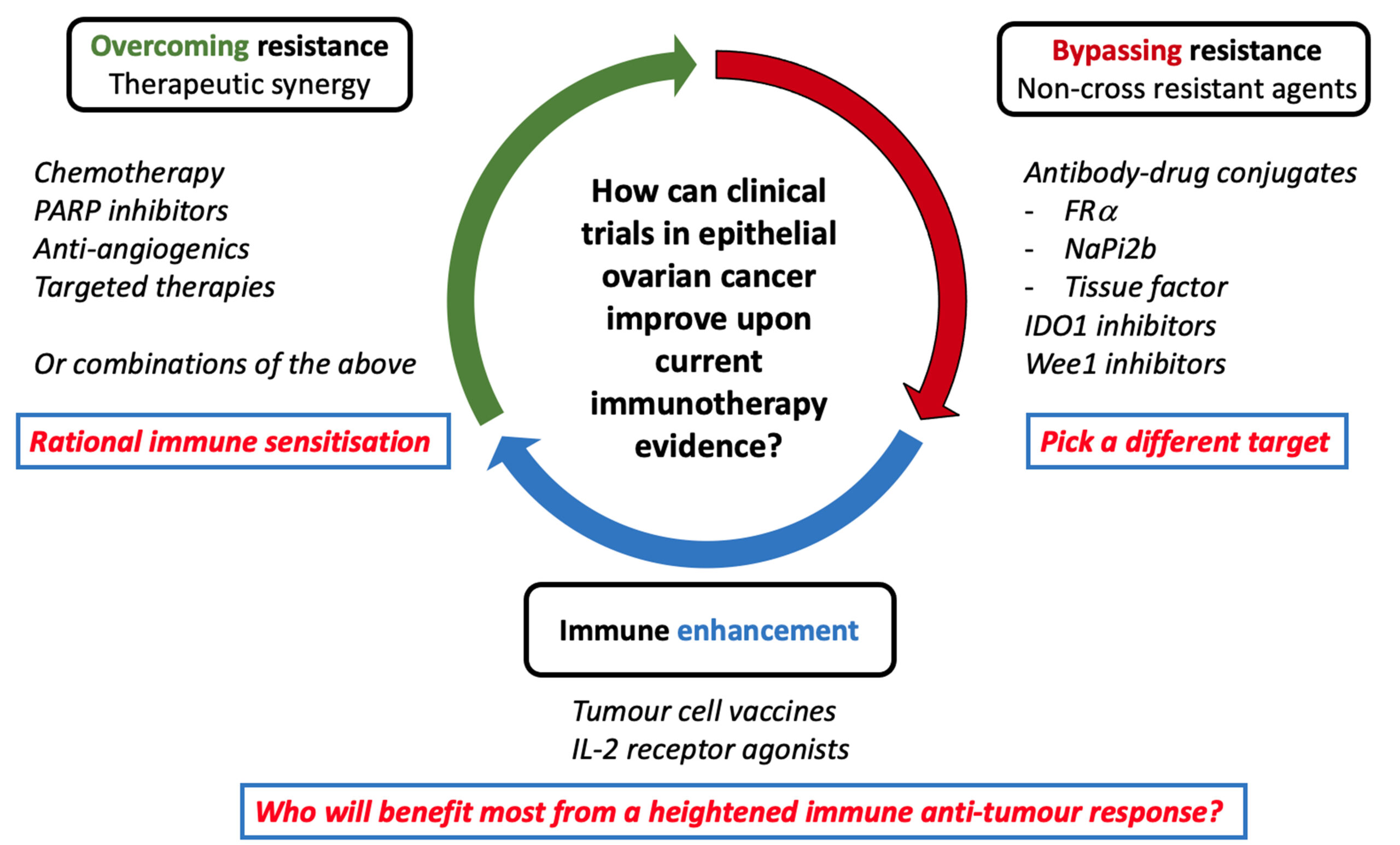
“PARP inhibitors, bevacizumab, and their mix are great alternatives, however they’re inadequate. There is severe reasoning and factor to include immune treatment to PARP inhibitors,” he stated. Monk likewise detailed 3 crucial systems supporting this method: PARP inhibitors’ disturbance with DNA repair work resulting in increased neoantigens, activation of the STING (stimulator of interferon gene) path, and increased PD-L1 expression.
Research Study Design and Patient Population
The trial registered 863 clients with FIGO phase III-IV AHGOC throughout 294 websites in 24 nations. Clients were arbitrarily appointed 1:1 to get either oral rucaparib 600 mg two times daily plus IV nivolumab 480 mg every 4 weeks or rucaparib plus placebo. The main endpoint was investigator-assessed, progression-free survival (PFS) in the ITT population. The detectives likewise carried out exploratory analyses to examine PFS in homologous recombination shortage (HRD) subgroups and PD-L1 subgroups.
Mix Therapy Is Not Superior to Monotherapy
Throughout his talk, Monk stated he was amazed that the PARP inhibitor and the immune checkpoint representative didn’t supply synergistic impacts equating into a low threat ratio, due to the fact that of the modes of action of the drugs.
“PARP inhibitors result in increased neoantigens, which implies more immunogenicity. We had actually hoped that we might enhance PFS. We were enthusiastic in attempting to target a risk ratio of 0.75 and increase the average PFS to 28 months,” he stated.
These findings disappointed the scientists’ high expectations, according to Philipp Harter, MD, PhD, of Kliniken Essen-Mitte in Essen, Germany.
“We wished to see equivalent outcomes like in cancer malignancy, cervic