— Different routines revealed equivalent pharmacokinetic and pharmacodynamic outcomes
by Judy George, Deputy Managing Editor, MedPage Today
October 29, 2024
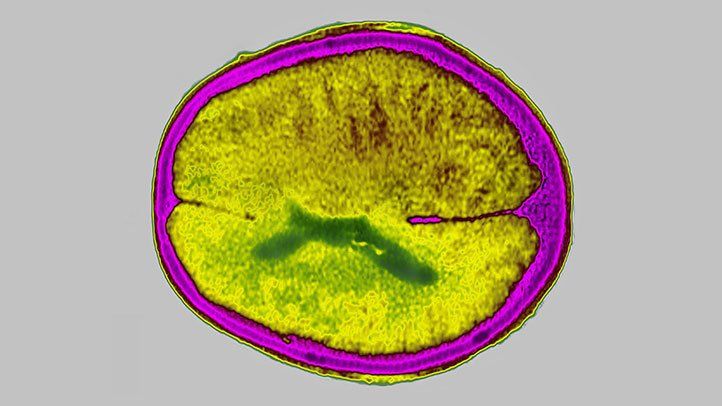
An alternate dosing prepare for the anti-amyloid drug donanemab (Kisunla) decreased the danger of brain edema and effusion however still preserved enough amyloid decrease in early Alzheimer’s illness, the stage IIIb TRAILBLAZER-ALZ 6 research study revealed.
At week 24, the frequency of amyloid-related imaging irregularities with edema and effusion (ARIA-E) was 23.7% for the basic dosing arm, and 18.6%, 18.3%, and 13.7% for each of 3 alternative dosing arms, reported John Sims, MD, of drugmaker Eli Lilly in Indianapolis, at the yearly Clinical Trials on Alzheimer’s Disease conference.
The basic dosing routine was 700 mg of donanemab for the very first 3 infusions, then 1,400 mg for each infusion after that. The customized titration arm with the most affordable ARIA-E frequency (13.7%) was 350 mg for the very first infusion, 700 mg for the 2nd infusion, 1,050 mg for the 3rd infusion, and 1,400 mg for each infusion after that.
The posterior likelihood of the customized titration arm accomplishing a minimum of a 20% relative threat decrease in ARIA-E versus the basic arm was 94.1%.
“We had the ability to show that a person of the dosing paradigms– the customized titration arm– did accomplish success in decreasing ARIA-E rates,” Sims stated. “That was accompanied by lower symptomatic frequencies, lower radiographic intensities, and a relatively remarkable effect on the[[APOE4]homozygous population.”
The customized titration and basic dosing programs had similar pharmacokinetic profiles and a similar pharmacodynamic result on amyloid and plasma p-tau217, he included.
TRAILBLAZER-ALZ 6 is still continuous however “offered the medical ramifications and the security findings of this, we prepare to send and discuss this with international regulators for a possible upgrade to labels,” Sims stated.
Donanemab was authorized by the FDA in July to deal with grownups with early symptomatic Alzheimer’s illness with verified am