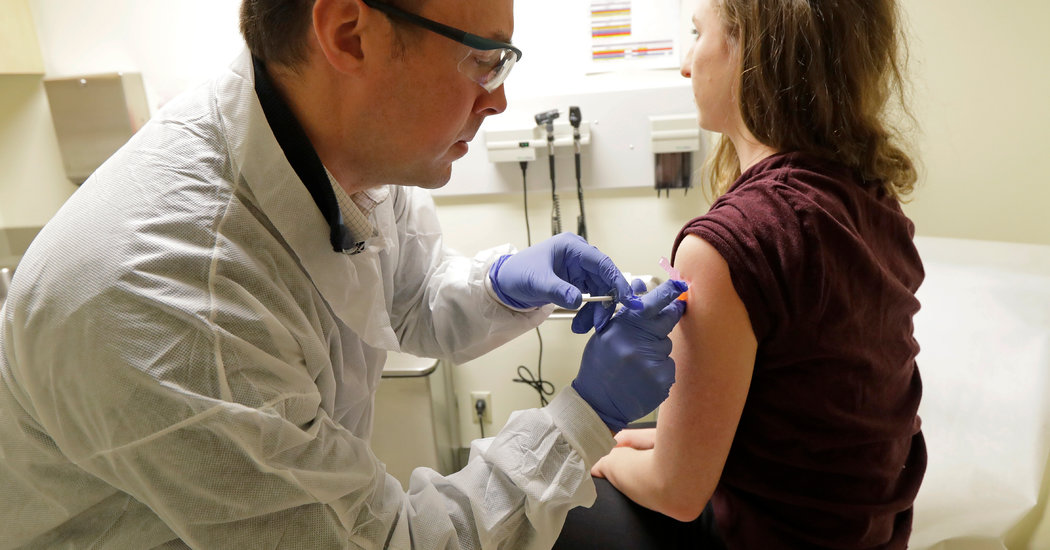
The very first testing in human beings of an experimental vaccine for the brand-new coronavirus began on Monday, the National Institute of Allergy and Infectious Diseases revealed.
The main objective of this first set of tests is to discover if the prospective vaccine is safe. If it is, later research study will figure out how well it works.
The trial was “ introduced in record speed,” Dr. Anthony Fauci, the institute’s director, said in a declaration.
Such fast development of a potential vaccine is unmatched, and it was possible because scientists had the ability to utilize what they currently learnt about associated coronaviruses that had caused other diseases outbreaks, SARS and MERS.
Regardless of the rapid development, even if the vaccine is shown safe and efficient against the virus, it will not be available for at least a year.
The tests, which are being carried out at Kaiser Permanente Washington Health Research Institute in Seattle, utilize a vaccine made by Moderna Inc
Seattle was selected as a test site prior to the United States had any recognized coronavirus cases, not because of the break out that appeared there. Washington State has been hard hit by the virus, with more than 670 cases to date.
Moderna utilizes genetic product– messenger RNA– to make vaccines, and the business has nine others in different phases of advancement, including numerous for infections that trigger respiratory health problems. No vaccine made with this innovation has actually yet reached the market.
The transmittable illness institute has actually been working with Moderna due to the fact that the RNA approach can produce vaccine extremely rapidly, stated Dr. Barney Graham, the deputy director of the institute’s Vaccine Research study.
He stated the researchers at the vaccine center were concentrated on pandemic readiness.
” The goal here is to be all set for all the infection households that can infect humans,” he said.
As bad as this epidemic is, Dr. Graham stated, in one way it is fortunate that a coronavirus triggered it, because the researchers were at least partly ready for it. If another type of virus had triggered the outbreak, it might have taken months longer to create a potential vaccine.
Other business, using various methods, are likewise trying to make coronavirus vaccines. Moderna is the first to reach a scientific trial.
The trial will register 45 healthy adults ages 18 to 55. Each will get two shots, 28 days apart. Moderna calls the vaccine mRNA-1273
Three various dosages will be checked– each in 15 individuals– and the individuals will be studied to determine whether the vaccine is safe and whether it promotes the body immune system to make antibodies that can stop the infection from replicating and prevent the illness it triggers.
4 individuals were immunized on Monday, and four more are to get shots on Tuesday. There will be a time out to monitor them, before more individuals receive injections, Dr. Graham said.
The individuals will be followed for a year, but Stéphane Bancel, the president of Moderna, said in an interview that security data would be offered a couple of weeks after the injections were provided. If the vaccine then appears safe, he said, Moderna will ask the Food and Drug Administration for consent to move ahead to the next phase of screening even prior to the very first phase is completed.
The 2nd round of testing, to measure effectiveness as well as to verify security, will consist of a lot more participants.
Moderna, with head office in Cambridge, Mass., and a manufacturing plant in neighboring Norwood, is already purchasing brand-new equipment so that it will able to produce countless doses. Mr. Bancel acknowledged that the company was taking a threat, since neither security nor efficacy has been shown yet.
” Human beings are suffering and time is of the essence,” he said. “Every day matters. We have taken these choices to take the threat, due to the fact that we believe it is the ideal thing to do.”
The company’s stock rate jumped in February in reaction to report about the vaccine. And on Monday, Moderna’s stock increased more than 24 percent, rising $5.19 to close at $2649
Deal with the vaccine started in January, as soon as Chinese scientists posted the genetic sequence of the new coronavirus on the web. Scientists at Moderna and the N