NEW DELHI: The race for an indigenous vaccine against the novel coronavirus is on in earnest. Human trials using the vaccine candidates of two companies — Bharat Biotech and Zydus Cadila — are currently on in six cities in as many states. The latest subject was a 30-year-old man who on Friday was the first person from Delhi to be given a 0.5 ml intramuscular injection of Bharat Biotech (BB)’s Covaxin at AIIMS.
Both BB and Zydus were granted permission for Phase I and II clinical trials and administered the first doses of their vaccine candidates to volunteers on July 15.
A third vaccine candidate, developed by Oxford University, is soon to be tested in India. Serum Institute, which is in a manufacturing partnership with the UK’s Astra Zeneca, has said it will begin human trials as soon as it receives regulatory approval.
Read our coronavirus live blog for all the latest news and updates
BB’s Covaxin, developed in collaboration with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV), will be tested at 12 hospitals — including AIIMS, Delhi and Patna, and PGI Rohtak — in 12 cities. The first phase will involve over 500 volunteers, all healthy and between the ages of 18 and 55 with no co-morbidities.
Testing of Zydus’s candidate, ZyCoV-D, is currently limited to its research centre in Ahmedabad, but will be extended to multiple cities.
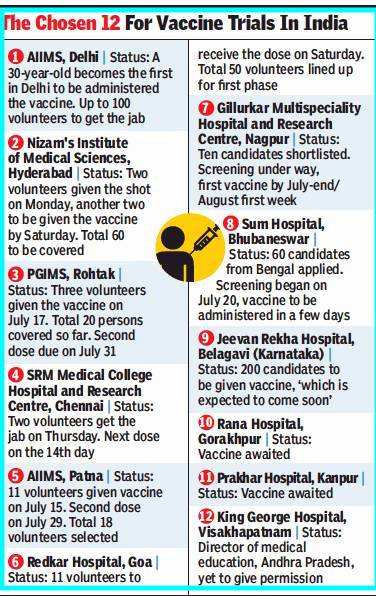
Covaxin trials have already begun in Hyderabad, Patna, Kancheepuram, Rohtak, and now Delhi, to be followed by Nagpur, Bhubaneshwar, Belgaum, Gorakhpur, Kanpur, Goa and Visakhapatnam.
Coronavirus outbreak: Complete coverage
At AIIMS, Delhi, Dr Sanjay Rai,