Working with little bacteria, Michigan Enlighten College researchers led by Lee Kroos non-public made a discovery that can non-public big implications for biology.
The researchers non-public published a brand sleek come that nature can inhibit or swap off important proteins identified as intramembrane proteases, which the crew reported April 26 within the journal eLife.
Even though the researchers made this discovering the utilization of a mannequin organism, a microbe identified as Bacillus subtilis, this form of protein is extremely conserved, which is how evolutionary biologists sigh, “or no longer it is all around the build apart.”
These kinds of proteases are found in organisms that span the kingdoms of life, from single-celled bacteria to americans. In truth, the first intramembrane protease became as soon as found in humans in 1997 and per chance the good-identified member of this family, named gamma-secretase, is implicated in Alzheimer’s disease.
“Our paper shows the first example of regulating an intramembrane protease with pure inhibitor proteins,” said Kroos, a professor within the School of Pure Science’s Department of Biochemistry and Molecular Biologyand Department of Microbiology and Molecular Genetics. “That affords us some tips on how we are going to likely be succesful of exercise and mimic that.
“Will it show us the good technique to modulate gamma-secretase? No,” Kroos said. “However it can perhaps give folks some tips on decorations they’d even build apart on inhibitors to test out as therapeutics.”
The exercise of this recordsdata to procure pills to cope with Alzheimer’s will preserve years, Kroos said, nonetheless the findings would possibly per chance perhaps non-public more quick impacts in preventing particularly spoiled and stubborn bacterial pathogens. That involves Bacillus anthracis, the bacterium gradual anthrax infections, and hundreds of bacteria accountable for tetanus, botulism and meals poisoning.
“Many, many bacteria non-public intramembrane proteases that are somewhat closely connected to the one we studied,” said Kroos, who has been a fellow of the American Association for the Pattern of Science since 2014, thanks in segment to his work furthering our figuring out of biology with bacteria. “If we figure these out, lets determine the good technique to procure bacteria less stress-resistant and more treatable with antibiotics.”
A much bigger figuring out of these proteases would possibly per chance perhaps also lend a hand make strategies in hundreds of areas, at the side of agriculture and environmental protection, eLife well-known in a digest featuring the researchers’ work. Beyond that, it helps paint a more total portray of how life works.
“This work must non-public a astronomical impact influencing our figuring out of the legislation of this class of proteins across the tree of life,” wrote Petra Anne Levin, an editor for eLife and a professor of biology at Washington College in St. Louis.
Scissors, spores and Corvettes
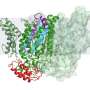
A protease is an enzyme, a form of protein machine, that nature makes exercise of to reduce up hundreds of proteins. Right here’s a elementary biological process that cells exercise to construct a diversity of targets. An intramembrane protease is an enzyme that has its active role—the role the build apart the enzyme does the snipping—buried inner a cell membrane.
“Every every so ceaselessly it is probably going you’ll hear them called ‘scissors within the membrane,'” Kroos said. “These intramembrane proteases attain in reality important issues in cells.”
The protease studied by the researchers, as an illustration, is segment of the biological plan that B. subtilis makes exercise of to procure spores when meals is scarce. Spores are really dormant cells covered in a protein armor that can climate harsh situations after which reactivate as soon as issues give a enhance to (hundreds of bacteria at the side of B. anthracis also make spores, which is one motive these pathogens are so persistent).
Because intramembrane proteases attain their work all around the confines of a cell membrane, or no longer it is been no longer easy for researchers to need precisely how they work. Along with to the complexity of the mission, the researchers conception their protease will be working in a cosmopolitan come that had by no manner been documented before.
“Whenever you glimpse at hundreds of connected organisms, you behold that this methodology has developed a lot,” Kroos said. “B. subtilis is esteem the Corvette. It has the excessive-stop equipment.”
Notion this excessive-stop equipment required intensive genetic and biochemical testing, which became as soon as led by Sandra Olenic, a doctoral pupil in Kroos’s lab. Olenic earned her Ph.D. after ending this mission and is now a postdoctoral pupil at Tufts College.
While Olenic designed and ran experiments, she