A faecal-transplant treatment called Rebyota has actually been authorized by the United States Food and Drug Administration. A single dosage can avoid a kind of reoccurring infection in the gut
By Grace Wade
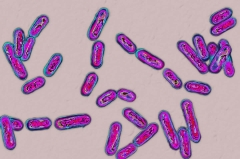
Clostridioides difficile germs seen under a microscopic lense BSIP SA/ Alamy Stock Photo
A drug called Rebyota has actually ended up being the very first faecal transplant item authorized for usage by the United States Food and Drug Administration (FDA). Established by Swiss business Ferring Pharmaceuticals, the treatment utilizes contributed human stool to avoid reoccurring Clostridioides difficile infections (CDI) in grownups.
Between 15,000 and 30,000 individuals in the United States pass away each year due to CDI, which takes place when the gut microbiome is interfered with, frequently by prescription antibiotics, enabling a toxin-producing germs referred to as C. difficile to increase. Signs consist of diarrhoea, stomach discomfort, fever and even organ failure. As much as 25 percent of individuals experience reoccurring infections after a very first C. difficile infection, and treatment choices are restricted.
Rebyota is a single-dose treatment administered through the anus. It utilizes contributed human stool to bring back the balance of germs in the gut of people who have actually currently finished antibiotic treatment for CDI.
In an eight-week trial of 262 grownups with frequent CDI, Rebyota avoided future infections in almost 71 percent of cases, whereas the exact same held true for less than 58 percent of those offered a placebo.
While donors and their stool are evaluated for pathogens, there is still a ri