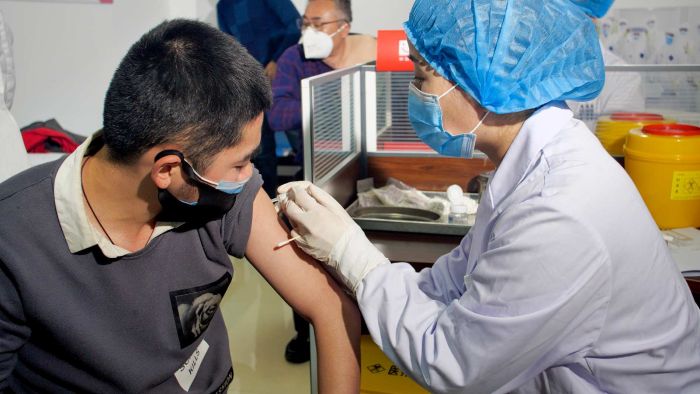
In a medical centre in the eastern city of Xuzhou, a few dozen healthy adults have become some of the first to trial a vaccine candidate for the coronavirus.
Key points:
- Five of the eight human trials to find a COVID-19 vaccine are in China
- Sinovac Biotech is trying to prevent the virus’ ability to reproduce
- Beijing is keen for a successful Chinese vaccine to help restore its global reputation
They are among a small group of people in China, the US and Britain who are pioneering the first human trials of different potential vaccines for COVID-19.
According to the Chinese company behind the Xuzhou vaccine, Sinovac Biotech, they are working around the clock.
“Normally the development of a vaccine will take eight to 10 years,” senior director of overseas business for Sinovac Meng Weining told the ABC.
“For this vaccine, it’s really a pandemic, so we’re trying our best to make it as quick as possible for each step.”
Sinovac — a private company supported by China’s government — previously worked on a SARS vaccine that was abandoned when the deadly virus disappeared in 2003, and in more recent years has developed avian flu and hepatitis vaccines.
This time, Sinovac is using a conventional method for its potential vaccine, inactivating the virus’ ability to reproduce.

It’s now one of five Chinese companies or government research organisatio