The state of Ohio has, as of this writing, five confirmed cases of Covid-19, the disease caused by the coronavirus SARS-CoV-2. If you ask Amy Acton, the director of the Ohio Department of Health, that’s the tip of the tip of a very dangerous iceberg. “We know now, just the fact of community spread says that at least 1 percent, at the very least 1 percent, of our population is carrying this virus in Ohio today,” Acton said at a press conference on Thursday. “We have 11.7 million people.” In other words, Acton was implying: 117,000 cases in Ohio alone.
How could Acton know this? Well, technically, she can’t. She was extrapolating from what little data scientists actually have about the spread of the pandemic. Officially, there have been just shy of 130,000 cases of Covid-19 on Earth, so a six-digit toll among Ohioans would be terrible, with extraordinary implications for how many cases might be out there undetected in the rest of the country.
What Is the Coronavirus?
Plus: How can I avoid catching it? Is Covid-19 more deadly than the flu? Our in-house Know-It-Alls answer your questions.
Whether that number is accurate or not is exactly the sort of thing public health workers would like to know. Who’s sick? How bad? How fast will the disease spread? But in the United States, nobody has good answers, for one simple but terrifying reason: There aren’t enough tests. Unlike for the flu, or previous coronaviruses like SARS or MERS, or sexually transmitted diseases, or a host of other infections, health workers have no way to find out whether a person sitting in front of them has Covid-19 or not. That technology actually exists and is relatively simple. They just don’t have access to it. Right now, four months into a global pandemic, two weeks since the first case of “community spread” within US borders, doctors and health workers have no way of testing everyone to see whether they have Covid-19.
The American Enterprise Institute’s program to estimate total US testing capacity currently puts that number at just over 22,000 tests a day—roughly twice the daily capacity of South Korea, a country with just one-sixth the population of the US and about one-thousandth the square mileage. That shortfall limits what scientists know about the disease—and therefore what they know about how to fight it. America is behind a fearsome curve, looking at a potential explosion of infection numbers. A large percentage of Americans will get sick enough to require hospitalization, and some of them are going to die. But nobody had enough tests in January or February to try to get ahead of that problem, and nobody has enough tests now to know how big a wave is yet to come. The reasons why are both scientific and political, and it’ll take deft science and politics to fix them.
Broadly, the response to an infectious disease outbreak has two phases: containment and mitigation. The containment phase happens early, when initial cases begin to appear. Public health workers tasked with surveillance do interviews to determine all the people an infected person might have come into contact with, to notify them and either isolate or treat them, in an effort to reduce further spread. But you can see where this is headed: Without the ability to test whether people are infected, health workers can’t effectively wall off these networks.
In the second phase, when the disease has spread beyond any public health system’s ability to investigate and handle individual cases, people need to understand what they’re up against. Without the ability to test vast numbers of people, it’s hard to calculate how many of the sick will need hospitalization, or will likely die. These numbers will be (figuratively and literally) all over the map. With Covid-19, the estimated percentage of infected people who die of the disease, or the “case fatality rate,” has varied from less than 1 percent to 15 percent, depending on the depth of testing conducted by each nation and how much data they actually released. Lots of factors might explain this variance, from a population’s overall wellness to its demographics to the nation’s health care system. It’d be nice to know which percentage is correct.
As the fight moves from individuals to entire populations, the goal of mitigation measures like social distancing, canceling large events, closing schools, and telling people to work from home is, sure, to lower the number of infections, but also to “flatten the curve,” as epidemiologists say. That curve is the number of sick people at a given time, and the best thing that could happen right now would be squishing that tsunami into a ripple, not necessarily reducing the overall number of infections but spacing them out, slowing them down. That’s because US hospitals have limited staff, equipment, and space, with just 2.9 hospital beds per 1,000 people. That’s fewer than Italy but more than Iran, whose health systems both got utterly slammed. And that’s also in the hope that, with more time to work the problem, new treatments might get figured out.
How Tests Work
When testing for a new virus like SARS-Cov-2, the first wave of diagnostics almost always relies on two important (though not particularly modern) technologies.
The first, PCR, or polymerase chain reaction, is a DNA amplification technique that is routinely used in the lab to turn tiny amounts of DNA into large enough quantities that they can be analyzed. Invented in the 1980s by Kary Mullis, the Nobel Prize-winning technique uses cycles of heating and cooling to make millions of copies of a very small amount of DNA. When combined with a fluorescent dye that glows in the presence of DNA, PCR can actually tell scientists how much DNA there is. That’s useful for detecting when a pathogen is present, either circulating in a host’s body or left behind on surfaces.
But if scientists want to detect a virus like SARS-CoV-2, they first have to turn its genome, which is made of single-stranded RNA, into DNA. They do that with a handy enzyme called reverse-transcriptase. Combine the two techniques and you’ve got RT-PCR.
Currently, RT-PCR is the only way to determine if a person has Covid-19. No other kinds of tests can yet distinguish the virus that causes it from influenza or the other dozen or so respiratory bugs that are circulating this time of year. “It’s a very standard, reliable technique used in microbiology labs almost everywhere that can be quickly applied to clinical testing,” says Louis Mansky, director for the Institute of Molecular Virology at the University of Minnesota. “It’s the fastest possible kind of test to develop.”
But until other kinds of tests can be developed and approved, all Covid-19 tests have to be conducted in a lab by trained technicians. They require PCR machines and people trained to use them, so they can’t be performed in a clinic or inside a patient’s home. Since PCR is such a workhorse of the biology world, lots of research labs at universities and hospitals have the necessary equipment and personnel, says Manksy. But in the US, only labs that have been certified by the federal Centers for Medicare and Medicaid Services can process clinical samples. That approval process can take months. “We have one of the more cumbersome—or, you could say, diligent—regulatory systems for testing,” says Bruce Carlson, publisher of Kalorama Information, a market research firm that covers medical diagnostics. “We’re very concerned about false positives, just as damning as false negatives.”
The test itself only takes about a day to run if you have all the required reagents. But shortages and shipping logistics can easily add days or even weeks to get a result. (This is, in fact, already happening, but more on that later.) Let’s start with what should happen:
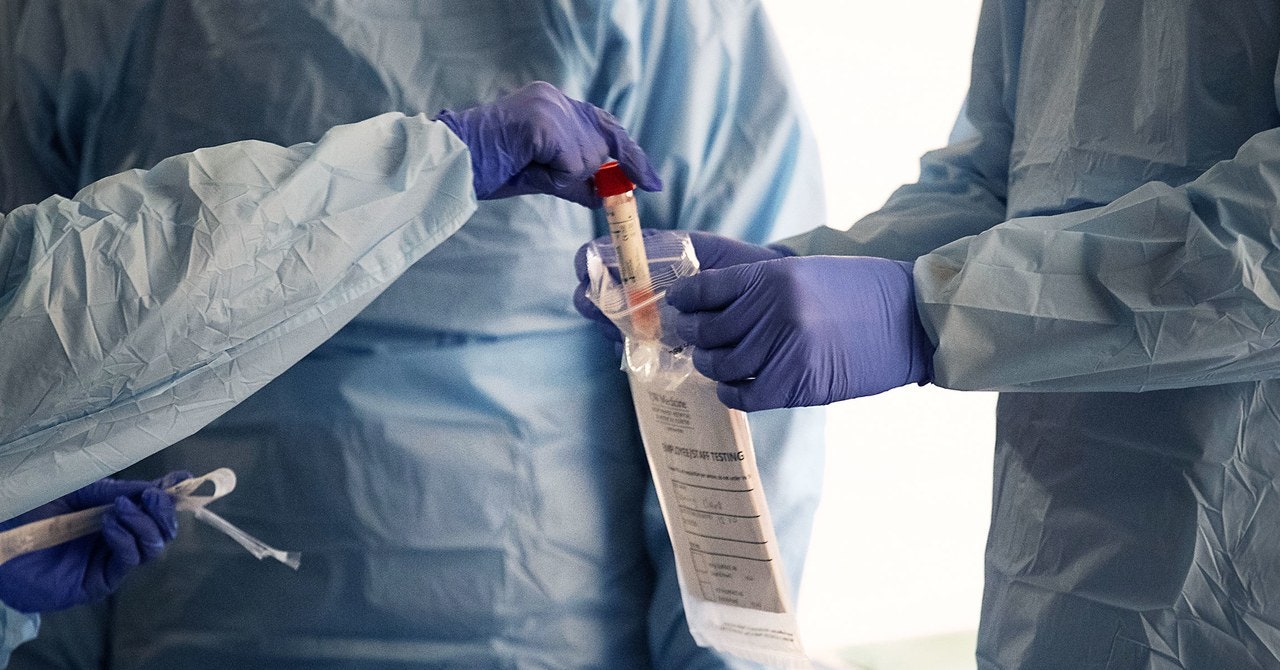
The very first step is collecting a sample. Using a sterile soft plastic stick, health care workers swab the inside of a patient’s nose or back of the throat. The goal is to collect material that’s recently been in the lungs, where the virus is believed to replicate. That stick then gets sealed up and shipped in a cold container to the testing lab. The sample has to stay between 35 and 40 degrees Fahrenheit, and if it’s not processed within four days it either goes into a freezer or gets thrown out.
Once in a lab, the first step is to separate out the RNA from everything else in the sample—human cells, proteins, enzymes that would chew up that viral genetic code. This is called RNA extraction. If you’re doing it by hand, this process usually involves adding chemicals and centrifuging the sample so the RNA winds up in a different layer from e