By David L. Chandler, Massachusetts Institute of Technology
June 22, 2022
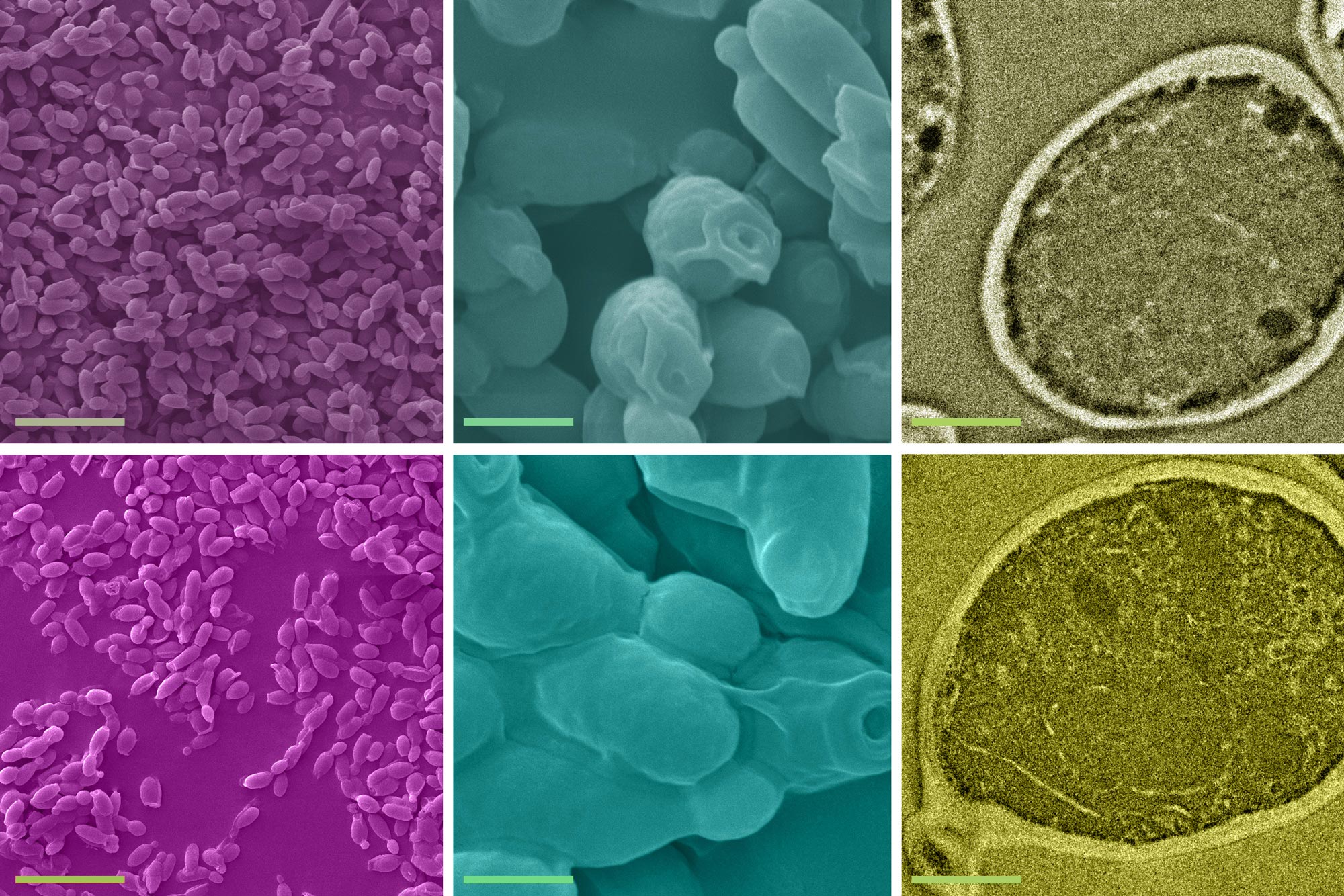
On this image, A relieve a watch on community of yeast cells (high row) is compared to yeast cells after they’ve gathered lead from unsuitable water (bottom row). Scanning electron microscope (SEM) photos indicate, at left, a high level figuring out, and at center, a more in-depth seek for at the yeast cells, and at honest transmission electron microscope (TEM) photos indicate an particular particular person yeast cell. Credit ranking: Courtesy of the researchers and edited by MIT News
A peculiar gape shows that yeast, an mighty waste product from breweries, can clear out even model amounts of lead.
Inactive yeast will be effective as an inexpensive, mighty, and uncomplicated field cloth for eradicating lead contamination from drinking water affords, per a weird evaluation by scientists at MIT’s Center for Bits and Atoms (CBA). The gape shows that this means will even additionally be efficient and economical, even down to share-per-billion phases of contamination. Excessive hurt to human properly being is identified to happen even at these low phases.
The device in which is highly efficient. In fact, the examine team has calculated that waste yeast discarded from a single brewery in Boston would ample to take care of town’s entire water offer. This form of fully sustainable diagram would now not finest purify the water however also divert what would otherwise be a waste circulation needing disposal.
The findings are detailed in the journal Nature Communications Earth and Atmosphere, in a paper by MIT Analysis Scientist Patritsia Statathou; Brown University postdoc and MIT Visiting Pupil Christos Athanasiou; MIT Professor Neil Gershenfeld, the director of CBA; and nine others at MIT, Brown, Wellesley College, Nanyang Technological University, and Nationwide Technical University of Athens. It became printed on June 13, 2022.
The team demonstrated that a single gram of slothful, dried yeast cells can procure up to 12 milligrams of lead in aqueous strategies with initial lead concentrations below 1 share per million. Besides they showed that the project is amazingly snappy, taking lower than 5 minutes to cease. Credit ranking: Courtesy of the researchers and edited by MIT News
Lead and diversified heavy metals in water are a indispensable global insist that continues to grow as a consequence of electronic waste and discharges from mining operations. In the U.S. on my own, better than 12,000 miles of waterways are impacted by acidic mine-drainage-water rich in heavy metals, the nation’s leading offer of water pollution. And in inequity to organic pollutants, most of that will even be in the end broken down, heavy metals don’t biodegrade, however persist indefinitely and bioaccumulate. They’re either very now not going or very expensive to utterly procure by venerable techniques such as chemical precipitation or membrane filtration.
Lead is extremely toxic, even at cramped concentrations, especially affecting kids as they grow. The European Union has reduced its fashioned for allowable lead in drinking water from 10 parts per billion to 5 parts per billion. In the U.S., the Environmental Security Agency has declared that no level at all in water affords is safe. And moderate phases in our bodies of flooring water globally are 10 times increased than they were 50 years previously, ranging from 10 parts per billion in Europe to loads of of parts per billion in South America.
“We don’t comely must prick the existence of lead; we must build away with it in drinking water,” says Stathatou. “And the real fact is that the venerable therapy processes are now not doing this effectively when the initial concentrations they’ve to procure are low, in the parts-per-billion scale and below. They either fail to utterly procure these model amounts, or in picture to assemble so they utilize loads of vitality and so they plot toxic byproducts.”
The solution studied by the MIT team is now not a weird one — a project called biosorption, in which slothful organic field cloth is archaic to procure heavy metals from water, has been identified for a pair of a protracted time. Nonetheless the project has been studied and characterized finest at mighty increased concentrations, at better than one share-per-million phases. “Our gape demonstrates that the project can certainly work efficiently at the mighty lower concentrations of fashioned exact-world water affords, and investigates intimately the mechanisms inquisitive regarding the project,” Athanasiou says.
The team studied the spend of a model of yeast widely archaic in brewing and in industrial processes, called S. cerevisiae, on pure water spiked with model amounts of lead. They demonstrated that a single gram of the slothful, dried yeast cells can procure up to 12 milligrams of lead in aqueous strategies with initial lead concentrations below 1 share per million. Besides they showed that the project is amazingly snappy, taking lower than 5 minutes to cease.
For the reason that yeast cells archaic in the project are slothful and desiccated, they require no explicit care, in inequity to diversified processes that depend on residing biomass to develop such functions which require vitamins and sunlight to accumulate up the affords filled with life. What’s extra, yeast is abundantly on hand already, as a waste product from beer brewing and from various diversified fermentation-essentially based industrial processes.
Stathatou has estimated that to keen a water offer for a city the size of Boston, which uses about 200 million gallons a day, would require about 20 a entire bunch yeast per day, or about 7,000 heaps per year. By comparison, one single brewery, the Boston Beer Company, generates 20,000 heaps a year of surplus yeast that is now not any longer precious for fermentation.
The researchers also carried out a sequence of checks to search out out that the yeast cells are to blame for biosorption. Athanasiou says that “exploring biosorption mechanisms at such now not easy concentrations is a cosmopolitan insist. We were the principle to spend a mechanics standpoint to unravel biosorption mechanisms, and we discovered that the mechanical properties of the yeast cells substitute very much after lead uptake. This offers basically unusual insights for the project.”
Devising a purposeful diagram for processing the water and retrieving the yeast, which would possibly perchance perchance then be separated from the lead for reuse, is the next stage of the team’s examine, they are saying.
“To scale up the project and if fact be told build it in discipline, it’s a must-own to embed these cells in a extra or less filter, and that’s the work that’s at point to ongoing,” Stathatou says. They’re also having a seek for at ways of getting better both the cells and the lead. “We own to behavior further experiments, however there is the probability to procure both back,” she says.
The identical field cloth can doubtlessly be archaic to procure diversified heavy metals, such as cadmium and copper, however that will require further examine to quantify the effective charges for those processes, the researchers philosophize.
“This examine published a if fact be told promising, cheaper, and environmentally pleasant solution for lead removal,” says Sivan Zamir, vice president of Xylem Innovation Labs, a water skills examine firm, who became now not associated to this examine. “It also deepened our working out of the biosorption project, paving the type for the reach of affords tailored to removal of diversified heavy metals.”
Reference: “Lead removal at model concentrations from water by slothful yeast cells” by Patritsia M. Stathatou, Christos E. Athanasiou, Marios Tsezos, John W. Goss, L. Camron Blackburn, Filippos Tourlomousis, Andreas Mershin, Brian W. Sheldon, Nitin P. Padture, Eric M. Darling, Huajian Gao and Neil Gershenfeld, 13 June 2022, Communications Earth & Atmosphere.
DOI: 10.1038/s43247-022-00463-0
The team also integrated Marios Tsezos at the Nationwide Technical University of Athens, in Greece; John Unsuitable at Wellesley College; Camron Blackburn, Filippos Tourlomousis, and Andreas Mershin at MIT’s CBA; Brian Sheldon, Nitin Padture, Eric Darling at Brown University; and Huajian Gao at Brown University and Nanyang Technological University, in Singapore.