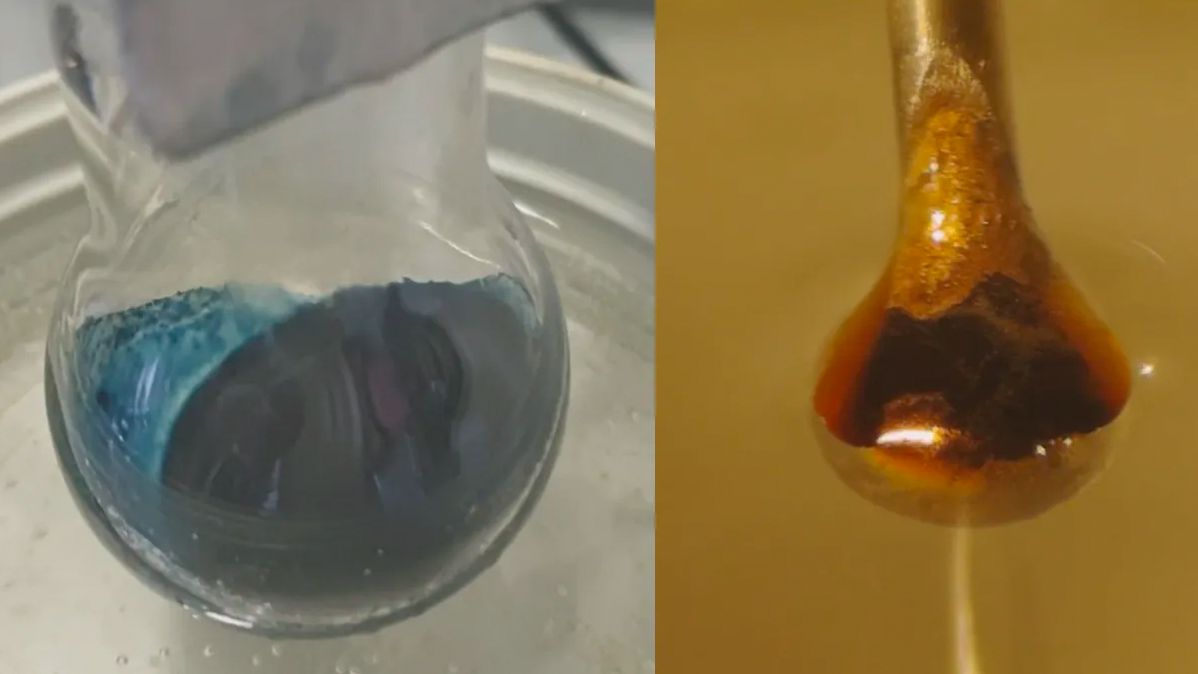Researchers may finally understand the strange transition behind a century-old chemistry experiment. The information of this change, in which including electrons to a brilliant blue ammonia option changes it into a shiny, metal bronze, have long avoided scientists.
The new research study reveals the subtle information of this change, and shows that this change is progressive, instead of abrupt. “What we’ve done effectively is that we have actually basically understood how these solutions behave at a vast array of concentrations utilizing a microjet technique,” said study co-author Ryan McMullen, a doctoral trainee in chemistry at the University of Southern California. This strategy, which involves shooting hair-thin streams of the service through a vacuum, has actually not been utilized on the glossy liquid before.
And the discovery might open brand-new kinds of reactions in natural chemistry in the future, McMullen told Live Science.
Related: 8 chemical components you never became aware of
Metals are a diverse group. Some, like lithium, are light enough to float, while others, like lead or osmium are exceptionally thick. Some need exceptionally heats to melt, while others melt quickly ( Mercury, for example, melts at minus 38.3 degrees Celsius, or minus 37.9 degrees Fahrenheit). Ultimately, what metals have in common is their ability to conduct electrical energy at absolute no, the point at which molecular movement from heat basically stops.
However how do some nonmetals transform into metals? In a new research study, scientists answered that question by including metals to liquid ammonia.
First, the scientists condensed ammonia, which is a gas at room temperature level, into a liquid by cooling it to negative 27.4 F (minus 33 C). They then included either salt, lithium or potassium, which are all alkali metals. (Rather famously, these metals react explosively when immersed in water.) The experiments were performed in partnership with researchers from the Czech Academy of Sciences and the Fritz-Haber Institute o