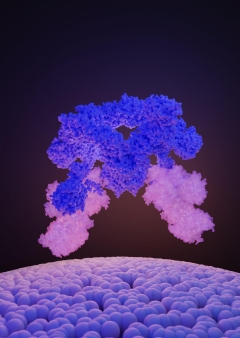
The SEA complex is made up of a cage-like core (SEACAT, blue) that controls the activity of the wings (SEACIT, white and brilliant). Credit: © Ciencia Graficada A UNIGE group has actually found the structure of a protein complex that manages the activity of the significant development regulator.The mTOR protein plays a main function in cell development, expansion, and survival. Its activity is impacted by the accessibility of nutrients in addition to numerous development elements such as hormonal agents. This protein has actually been connected to a variety of illness, consisting of cancer, where its activity often increases. A group from the University of Geneva (UNIGE), in partnership with scientists from the Martin Luther University (MLU) of Halle-Wittenberg in Germany, and the just recently inaugurated Dubochet Center for Imaging (UNIGE-UNIL-EPFL), has actually recognized the structure of the SEA complex, a synergistic set of proteins accountable for managing mTOR. The finding of this structure offers a much better understanding of how cells view nutrient levels in order to manage their development. The research study was just recently released in the journal Nature. From yeast to human beings, the mTOR protein (mammalian target of rapamycin) is the main controller of cell development. This protein responds to ecological hints such as nutrients and hormonal agents and controls a number of essential cellular functions such as protein and lipid synthesis, energy production by mitochondria, and cell structure company. mTOR activity interruptions are the origin of lots of conditions, consisting of diabetes, weight problems, epilepsy, and a number of kinds of cancer. 2 opposing functions in the exact same complexThe lab of Robbie Loewith, Professor in the Department of Molecular and Cellular Biology at the UNIGE Faculty of Science and director of the National Center for Competence in Research in Chemical Biology, has an interest in the guideline of mTOR, and in specific in the SEA complex, which is the direct sensing unit of nutrients and which manages the activity of mTOR. The SEA complex is made up of 8 proteins. One part of the SEA complex (SEACIT) is associated with the inhibition of mTOR activity, while the other part (SEACAT) is associated with its activation. In the lack of nutrients, the mTOR protein is obstructed by the SEACIT subcomplex, and cell development is hence avoided. On the other hand, in the existence of nutrients, the SEACAT subcomplex is believed to hinder the SEACIT subcomplex, which can no longer obstruct the mTOR protein. The main controller can then apply its triggering function in cell development by, for instance, promoting the production of proteins and lipids. How SEACAT manages SEACIT is still not comprehended. Identifying structure to comprehend the functionTo figure out the interactions in between the proteins of the SEA complex, and therefore much better comprehend how they work, the scientists set out to figure out the structure of this complex. After biochemically separating the SEA complex from all of the other parts in the cell, the researchers utilized the innovations of the Dubochet Center for Imaging of UNIGE, UNIL, and EPFL to get its molecular structure by cryo-electron microscopy (cryo-EM). “By freezing the samples extremely rapidly at -180 ° C, cryo-EM enables to get the structure of the proteins in their initial state, i.e. in their practical three-dimensional type,” discusses Lucas Tafur, a scientist in the Department of Molecular and Cellular Biology and very first author of the research study. SEACAT is required however not sufficientThe biochemical activities of the various elements of the complex were then evaluated in the lab. Regardless of the SEACAT subcomplex remaining in an active kind (as when in the existence of nutrients), the scientists observed that the SEACIT subcomplex is still active and efficient in obstructing mTOR. “This outcome is extremely unanticipated given that SEACAT has actually long been referred to as the direct inhibitor of SEACIT. We, for that reason, anticipated SEACIT to be non-active in the existence of active SEACAT. Our outcomes reveal that SEACAT acts more as a scaffold for the recruitment of other regulative proteins which its existence is, for that reason, essential however not enough for the inhibition of SEACIT,” discusses Robbie Loewith, the last author of the research study. Acquiring the structure of the SEA complex has actually enabled highlighting missing out on links in the mTOR regulative waterfall. “Obviously, we now require to recognize the as-yet unidentified partners that connect with this complex. These brand-new elements might show to be healing targets for growths where mTOR activity is exacerbated,” concludes Lucas Tafur. Referral: “Cryo-EM structure of the SEA complex” by Lucas Tafur, Kerstin Hinterndorfer, Caroline Gabus, Chiara Lamanna, Ariane Bergmann, Yashar Sadian, Farzad Hamdi, Fotis L. Kyrilis, Panagiotis L. Kastritis and Robbie Loewith, 26 October 2022, Nature. DOI: 10.1038/ s41586-022-05370 -0
Read More
Secret Regulator of Cell Growth Deciphered