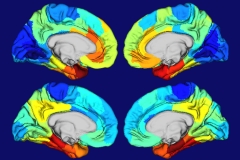
Red and orange locations on these heat maps of human brains reveal where the gene APOE is most active (leading 2 brain images) and where tangles of the protein tau are most focused (bottom 2 brain images). APOE is the greatest hereditary danger aspect for Alzheimer’s, and tau tangles drive mental retardation in the illness. The resemblances in the 2 sets of maps recommended to scientists at Washington University School of Medicine in St. Louis that APOE contributes in making sure brain locations especially susceptible to Alzheimer’s damage. Credit: Diana Hobbs/Washington University Research findings might assist describe uncommon signs such as issues with language and vision. The very first indication of Alzheimer’s illness is generally amnesia, followed by confusion and trouble believing. These signs show the normal pattern of gradually intensifying damage to brain tissues. Harmful clusters of proteins very first concentrate in the temporal lobes of the brain– the memory location– prior to infecting parts of the brain crucial for believing and preparing. A brand-new research study yields ideas regarding why particular parts of the brain are especially susceptible to Alzheimer’s damage. It boils down to the gene APOE, the best hereditary threat element for Alzheimer’s illness. The scientists, from Washington University School of Medicine in St. Louis, discovered that the parts of the brain where APOE is most active are the locations that sustain one of the most damage. Released on November 16 in the journal Science Translational Medicine, the findings assist discuss why signs of Alzheimer’s illness in some cases differ. They likewise highlight an understudied element of Alzheimer’s illness that recommends yet-to-be-discovered biological systems might play an essential function in the illness. “There are some uncommon, irregular kinds of Alzheimer’s in which individuals very first establish language or vision issues instead of memory issues,” stated senior author Brian A. Gordon, PhD, an assistant teacher of radiology at the School of Medicine’s Mallinckrodt Institute of Radiology. “When you scan their brains, you see damage to the language or the visual locations, and not a lot to the memory locations. Individuals with irregular Alzheimer’s are frequently evaluated out of research study studies since it’s simpler to study a group where everybody has the exact same set of signs. This heterogeneity informs us that there are things we still do not comprehend about how and why Alzheimer’s establishes the method it does. There’s a factor why particular brain locations end up being harmed and not others, and we do not understand that factor. Every secret we reveal with this illness presses us closer to what we require to resolve it.” Alzheimer’s illness starts with a brain protein called amyloid beta. The protein begins developing into plaques twenty years or more prior to individuals reveal the very first indications of neurological issues. After years of amyloid build-up, tangles of tau– another brain protein– start to form. Right after, tissues in the impacted locations start to wither and pass away, and cognitive decrease sets in. To comprehend why Alzheimer’s brain damage takes place where it does, Gordon and associates– consisting of very first author Aylin Dincer, a service technician in Gordon’s laboratory– studied 350 individuals who offer for memory and aging research studies through the School of Medicine’s Charles F. and Joanne Knight Alzheimer Disease Research. The individuals went through brain scans so the scientists might determine the quantity and area of amyloid plaques and tau tangles, and the volumes of numerous brain locations. Patterns of protein clumps and tissue damage in the volunteers were compared to the gene expression patterns of APOE and other genes related to Alzheimer’s illness as portrayed in the Allen Human Brain Atlas, a comprehensive map of gene expression in the human brain put together by the Allen Institute for Brain Sciences. “There was a close match in between where you see high APOE expression, and where you see tau tangles and tissue damage,” stated Gordon, likewise an assistant teacher of mental & brain sciences. “And not simply APOE. If you take a look at, state, the top 20 genes related to Alzheimer’s illness, they are all revealed in the temporal lobes in comparable patterns. There’s something essentially various about these areas that make them susceptible to Alzheimer’s mental retardation, which distinction is most likely baked in from birth and affected by an individual’s genes.” Everybody brings some variation of the APOE gene, however individuals who bring the APOE4 version depend on 12 times most likely to establish Alzheimer’s illness than the basic population, and at a more youthful age. Alzheimer’s scientists have actually long understood that APOE4 increases the build-up of amyloid beta in individuals’s brains. Studying mice that establish tau tangles however not amyloid plaques, David Holtzman, MD, the Barbara Burton and Reuben M. Morriss III Distinguished Professor of Neurology, and coworkers revealed that APOE4 likewise increases damage due to tau, even without amyloid present. To evaluate the result of the high-risk version of APOE on tau-related mental retardation in individuals, the scientists categorized each individual as bring the high-risk version or not, and evaluated the protein clusters and atrophy in their brains. “APOE4 providers are most likely to begin collecting amyloid, which puts them on the course to Alzheimer’s,” Gordon stated. “Then, for the exact same quantity of amyloid they get more tau tangles, which causes more atrophy. It’s a double hit on the brain.” In future work, Gordon and coworkers prepare to check out how patterns of gene expression associate with patterns of tau damage in individuals with irregular Alzheimer’s. “When we see somebody who provides with vision issues, exists a particular hereditary signature that represents the locations that are harmed in the brain?” Gordon asked. “We need to know why some individuals have these transformed patterns and what it suggests about how Alzheimer’s illness establishes and how it can be dealt with.” Referral: “APOE e4 genotype, amyloid-ß, and sex connect to anticipate tau in areas of high APOE mRNA expression” by Aylin Dincer, Charles D. Chen, Nicole S. McKay, Lauren N. Koenig, Austin McCullough, Shaney Flores, Sarah J. Keefe, Stephanie A. Schultz, Rebecca L. Feldman, Nelly Joseph-Mathurin, Russ C. Hornbeck, Carlos Cruchaga, Suzanne E. Schindler, David M. Holtzman, John C. Morris, Anne M. Fagan, Tammie L.S. Benzinger and Brian A. Gordon, 16 November 2022, Science Translational Medicine. DOI: 10.1126/ scitranslmed.abl7646 Funding: NIH/National Institutes of Health, Alzheimer’s Association, National Science Foundation
Read More
Why Alzheimer’s Disease Damages Certain Parts of the Brain– New Genetic Clues